
SL Paper 2
Chlorine undergoes many reactions.
of manganese(IV) oxide was added to of .
Chlorine gas reacts with water to produce hypochlorous acid and hydrochloric acid.
is a common chlorofluorocarbon, .
State the full electron configuration of the chlorine atom.
State, giving a reason, whether the chlorine atom or the chloride ion has a larger radius.
Outline why the chlorine atom has a smaller atomic radius than the sulfur atom.
The mass spectrum of chlorine is shown.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Outline the reason for the two peaks at and .
Explain the presence and relative abundance of the peak at .
Calculate the amount, in , of manganese(IV) oxide added.
Determine the limiting reactant, showing your calculations.
Determine the excess amount, in , of the other reactant.
Calculate the volume of chlorine, in , produced if the reaction is conducted at standard temperature and pressure (STP). Use section 2 of the data booklet.
State the oxidation state of manganese in and .
Deduce, referring to oxidation states, whether is an oxidizing or reducing agent.
Hypochlorous acid is considered a weak acid. Outline what is meant by the term weak acid.
State the formula of the conjugate base of hypochlorous acid.
Calculate the concentration of in a solution with a .
State the type of reaction occurring when ethane reacts with chlorine to produce chloroethane.
Predict, giving a reason, whether ethane or chloroethane is more reactive.
Write the equation for the reaction of chloroethane with a dilute aqueous solution of sodium hydroxide.
Deduce the nucleophile for the reaction in d(iii).
Ethoxyethane (diethyl ether) can be used as a solvent for this conversion. Draw the structural formula of ethoxyethane
Deduce the number of signals and their chemical shifts in the spectrum of ethoxyethane. Use section 27 of the data booklet.
Calculate the percentage by mass of chlorine in .
Comment on how international cooperation has contributed to the lowering of emissions responsible for ozone depletion.
This question is about carbon and chlorine compounds.
Ethane, C2H6, reacts with chlorine in sunlight. State the type of this reaction and the name of the mechanism by which it occurs.
Formulate equations for the two propagation steps and one termination step in the formation of chloroethane from ethane.
One possible product, X, of the reaction of ethane with chlorine has the following composition by mass:
carbon: 24.27%, hydrogen: 4.08%, chlorine: 71.65%
Determine the empirical formula of the product.
The mass and 1HNMR spectra of product X are shown below. Deduce, giving your reasons, its structural formula and hence the name of the compound.
Chloroethene, C2H3Cl, can undergo polymerization. Draw a section of the polymer with three repeating units.
The rate of the acid-catalysed iodination of propanone can be followed by measuring how the concentration of iodine changes with time.
I2(aq) + CH3COCH3(aq) → CH3COCH2I(aq) + H+(aq) + I−(aq)
Suggest how the change of iodine concentration could be followed.
A student produced these results with [H+] = 0.15 moldm−3. Propanone and acid were in excess and iodine was the limiting reagent.
Determine the relative rate of reaction when [H+] = 0.15 moldm−3.
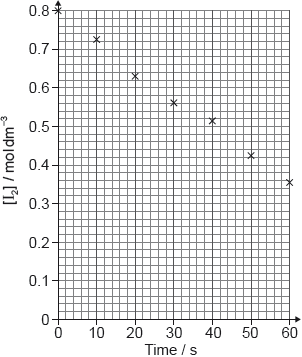
The student then carried out the experiment at other acid concentrations with all other conditions remaining unchanged.
State and explain the relationship between the rate of reaction and the concentration of acid.
There are many oxides of silver with the formula AgxOy. All of them decompose into their elements when heated strongly.
After heating 3.760 g of a silver oxide 3.275 g of silver remained. Determine the empirical formula of AgxOy.
Suggest why the final mass of solid obtained by heating 3.760 g of AgxOy may be greater than 3.275 g giving one design improvement for your proposed suggestion. Ignore any possible errors in the weighing procedure.
Naturally occurring silver is composed of two stable isotopes, 107Ag and 109Ag.
The relative atomic mass of silver is 107.87. Show that isotope 107Ag is more abundant.
Some oxides of period 3, such as Na2O and P4O10, react with water. A spatula measure of each oxide was added to a separate 100 cm3 flask containing distilled water and a few drops of bromothymol blue indicator.
The indicator is listed in section 22 of the data booklet.
Deduce the colour of the resulting solution and the chemical formula of the product formed after reaction with water for each oxide.
Explain the electrical conductivity of molten Na2O and P4O10.
Outline the model of electron configuration deduced from the hydrogen line emission spectrum (Bohr’s model).
The reactivity of organic compounds depends on the nature and positions of their functional groups.
The structural formulas of two organic compounds are shown below.
Deduce the type of chemical reaction and the reagents used to distinguish between these compounds.
State the observation expected for each reaction giving your reasons.
Deduce the number of signals and the ratio of areas under the signals in the 1H NMR spectra of the two compounds.
Explain, with the help of equations, the mechanism of the free-radical substitution reaction of ethane with bromine in presence of sunlight.
A student titrated an ethanoic acid solution, CH3COOH (aq), against 50.0 cm3 of 0.995 mol dm–3 sodium hydroxide, NaOH (aq), to determine its concentration.
The temperature of the reaction mixture was measured after each acid addition and plotted against the volume of acid.
Curves X and Y were obtained when a metal carbonate reacted with the same volume of ethanoic acid under two different conditions.
Using the graph, estimate the initial temperature of the solution.
Determine the maximum temperature reached in the experiment by analysing the graph.
Calculate the concentration of ethanoic acid, CH3COOH, in mol dm–3.
Determine the heat change, q, in kJ, for the neutralization reaction between ethanoic acid and sodium hydroxide.
Assume the specific heat capacities of the solutions and their densities are those of water.
Calculate the enthalpy change, ΔH, in kJ mol–1, for the reaction between ethanoic acid and sodium hydroxide.
Explain the shape of curve X in terms of the collision theory.
Suggest one possible reason for the differences between curves X and Y.
The structure of an organic molecule can help predict the type of reaction it can undergo.
Improvements in instrumentation have made identification of organic compounds routine.
The empirical formula of an unknown compound containing a phenyl group was found to be C4H4O. The molecular ion peak in its mass spectrum appears at m/z = 136.
The Kekulé structure of benzene suggests it should readily undergo addition reactions.
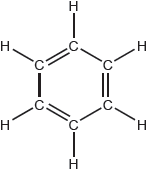
Discuss two pieces of evidence, one physical and one chemical, which suggest this is not the structure of benzene.
Formulate the ionic equation for the oxidation of propan-1-ol to the corresponding aldehyde by acidified dichromate(VI) ions. Use section 24 of the data booklet.
The aldehyde can be further oxidized to a carboxylic acid.
Outline how the experimental procedures differ for the synthesis of the aldehyde and the carboxylic acid.
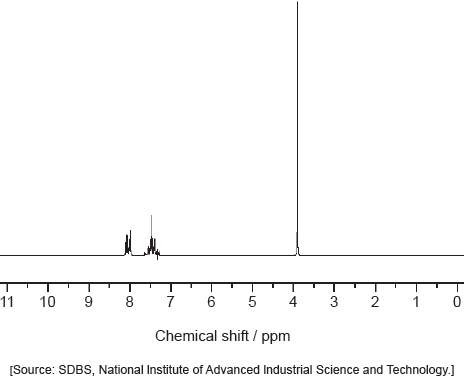
Deduce the molecular formula of the compound.
Identify the bonds causing peaks A and B in the IR spectrum of the unknown compound using section 26 of the data booklet.
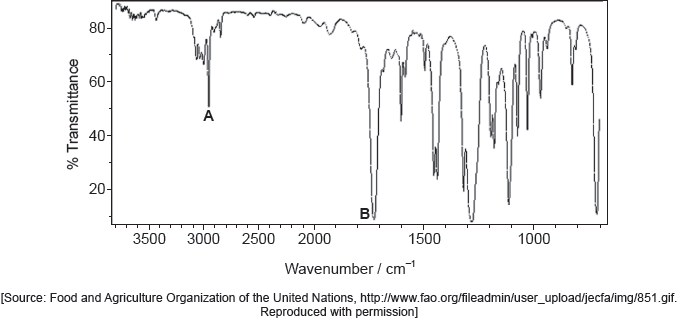
Deduce full structural formulas of two possible isomers of the unknown compound, both of which are esters.
Deduce the formula of the unknown compound based on its 1H NMR spectrum using section 27 of the data booklet.
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
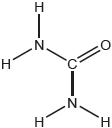
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) (H2N)2CO(g) + H2O(g) ΔH < 0
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
The mass spectrum of urea is shown below.
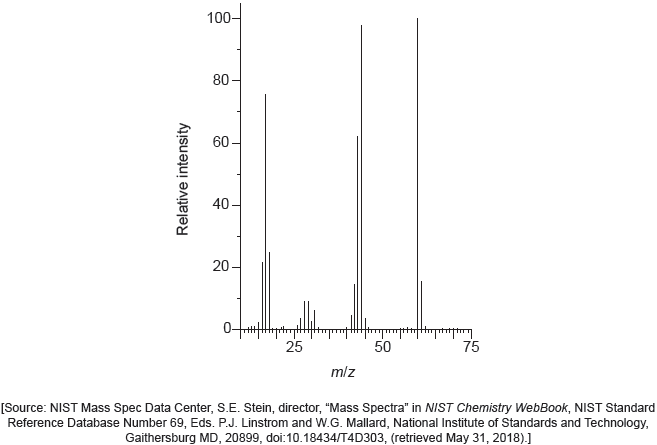
Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.
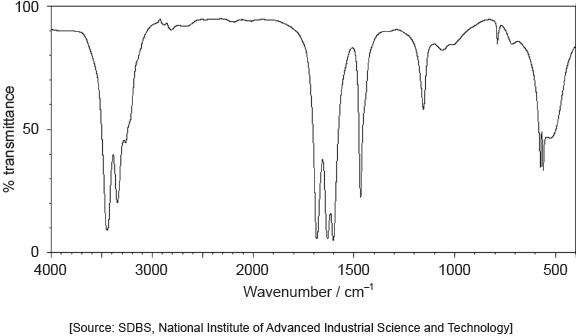
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Ethanol is obtained by the hydration of ethene, C2H4.
State the class of compound to which ethene belongs.
State the molecular formula of the next member of the homologous series to which ethene belongs.
Justify why ethene has only a single signal in its 1H NMR spectrum.
Suggest two possible products of the incomplete combustion of ethene that would not be formed by complete combustion.
A white solid was formed when ethene was subjected to high pressure.
Deduce the type of reaction that occurred.
Organic chemistry can be used to synthesize a variety of products.
Combustion analysis of an unknown organic compound indicated that it contained only carbon, hydrogen and oxygen.
Several compounds can be synthesized from but-2-ene. Draw the structure of the final product for each of the following chemical reactions.
Determine the change in enthalpy, ΔH, for the combustion of but-2-ene, using section 11 of the data booklet.
CH3CH=CHCH3 (g) + 6O2 (g) → 4CO2 (g) + 4H2O (g)
Write the equation and name the organic product when ethanol reacts with methanoic acid.
Oxidation of ethanol with potassium dichromate, K2Cr2O7, can form two different organic products. Determine the names of the organic products and the methods used to isolate them.
Deduce two features of this molecule that can be obtained from the mass spectrum. Use section 28 of the data booklet.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
Identify the bond responsible for the absorption at A in the infrared spectrum. Use section 26 of the data booklet.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
Deduce the identity of the unknown compound using the previous information, the 1H NMR spectrum and section 27 of the data booklet.
SDBS, National Institute of Advanced Industrial Science and Technology (AIST).
3.26 g of iron powder are added to 80.0 cm3 of 0.200 mol dm−3 copper(II) sulfate solution. The following reaction occurs:
Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s)
Determine the limiting reactant showing your working.
The mass of copper obtained experimentally was 0.872 g. Calculate the percentage yield of copper.
The reaction was carried out in a calorimeter. The maximum temperature rise of the solution was 7.5 °C.
Calculate the enthalpy change, ΔH, of the reaction, in kJ, assuming that all the heat released was absorbed by the solution. Use sections 1 and 2 of the data booklet.
State another assumption you made in (b)(i).
The only significant uncertainty is in the temperature measurement.
Determine the absolute uncertainty in the calculated value of ΔH if the uncertainty in the temperature rise was ±0.2 °C.
Sketch a graph of the concentration of iron(II) sulfate, FeSO4, against time as the reaction proceeds.
Outline how the initial rate of reaction can be determined from the graph in part (c)(i).
Explain, using the collision theory, why replacing the iron powder with a piece of iron of the same mass slows down the rate of the reaction.
When heated in air, magnesium ribbon reacts with oxygen to form magnesium oxide.
The reaction in (a)(i) was carried out in a crucible with a lid and the following data was recorded:
Mass of crucible and lid = 47.372 ±0.001 g
Mass of crucible, lid and magnesium ribbon before heating = 53.726 ±0.001 g
Mass of crucible, lid and product after heating = 56.941 ±0.001 g
When magnesium is burnt in air, some of it reacts with nitrogen to form magnesium nitride according to the equation:
3 Mg (s) + N2 (g) → Mg3N2 (s)
The presence of magnesium nitride can be demonstrated by adding water to the product. It is hydrolysed to form magnesium hydroxide and ammonia.
Most nitride ions are 14N3–.
Write a balanced equation for the reaction that occurs.
State the block of the periodic table in which magnesium is located.
Identify a metal, in the same period as magnesium, that does not form a basic oxide.
Calculate the amount of magnesium, in mol, that was used.
Determine the percentage uncertainty of the mass of product after heating.
Assume the reaction in (a)(i) is the only one occurring and it goes to completion, but some product has been lost from the crucible. Deduce the percentage yield of magnesium oxide in the crucible.
Evaluate whether this, rather than the loss of product, could explain the yield found in (b)(iii).
Suggest an explanation, other than product being lost from the crucible or reacting with nitrogen, that could explain the yield found in (b)(iii).
Calculate coefficients that balance the equation for the following reaction.
__ Mg3N2 (s) + __ H2O (l) → __ Mg(OH)2 (s) + __ NH3 (aq)
Determine the oxidation state of nitrogen in Mg3N2 and in NH3.
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
State the number of subatomic particles in this ion.
Some nitride ions are 15N3–. State the term that describes the relationship between 14N3– and 15N3–.
The nitride ion and the magnesium ion are isoelectronic (they have the same electron configuration). Determine, giving a reason, which has the greater ionic radius.
Suggest two reasons why atoms are no longer regarded as the indivisible units of matter.
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.
Lithium reacts with water to form an alkaline solution.
A 0.200 g piece of lithium was placed in 500.0 cm3 of water.
Determine the coefficients that balance the equation for the reaction of lithium with water.
Calculate the molar concentration of the resulting solution of lithium hydroxide.
Calculate the volume of hydrogen gas produced, in cm3, if the temperature was 22.5 °C and the pressure was 103 kPa. Use sections 1 and 2 of the data booklet.
Suggest a reason why the volume of hydrogen gas collected was smaller than predicted.
The reaction of lithium with water is a redox reaction. Identify the oxidizing agent in the reaction giving a reason.
Describe two observations that indicate the reaction of lithium with water is exothermic.
Sodium thiosulfate solution reacts with dilute hydrochloric acid to form a precipitate of sulfur at room temperature.
Na2S2O3 (aq) + 2HCl (aq) → S (s) + SO2 (g) + 2NaCl (aq) + X
Identify the formula and state symbol of X.
Suggest why the experiment should be carried out in a fume hood or in a well-ventilated laboratory.
The precipitate of sulfur makes the mixture cloudy, so a mark underneath the reaction mixture becomes invisible with time.
10.0 cm3 of 2.00 mol dm-3 hydrochloric acid was added to a 50.0 cm3 solution of sodium thiosulfate at temperature, T1. Students measured the time taken for the mark to be no longer visible to the naked eye. The experiment was repeated at different concentrations of sodium thiosulfate.
Show that the hydrochloric acid added to the flask in experiment 1 is in excess.
Draw the best fit line of against concentration of sodium thiosulfate on the axes provided.
A student decided to carry out another experiment using 0.075 mol dm-3 solution of sodium thiosulfate under the same conditions. Determine the time taken for the mark to be no longer visible.
An additional experiment was carried out at a higher temperature, T2.
(i) On the same axes, sketch Maxwell–Boltzmann energy distribution curves at the two temperatures T1 and T2, where T2 > T1.
(ii) Explain why a higher temperature causes the rate of reaction to increase.
Suggest one reason why the values of rates of reactions obtained at higher temperatures may be less accurate.
The Bombardier beetle sprays a mixture of hydroquinone and hydrogen peroxide to fight off predators. The reaction equation to produce the spray can be written as:
| C6H4(OH)2(aq) + H2O2(aq) | → | C6H4O2(aq) + 2H2O(l) |
| hydroquinone | quinone |
Calculate the enthalpy change, in kJ, for the spray reaction, using the data below.
The energy released by the reaction of one mole of hydrogen peroxide with hydroquinone is used to heat 850 cm3 of water initially at 21.8°C. Determine the highest temperature reached by the water.
Specific heat capacity of water = 4.18 kJkg−1K−1.
(If you did not obtain an answer to part (i), use a value of 200.0 kJ for the energy released, although this is not the correct answer.)
Identify the species responsible for the peak at m/z = 110 in the mass spectrum of hydroquinone.
Identify the highest m/z value in the mass spectrum of quinone.
Carbon forms many compounds.
C60 and diamond are allotropes of carbon.
But-2-ene reacts with hydrogen bromide.
Chlorine reacts with methane.
CH4 (g) + Cl2 (g) → CH3Cl (g) + HCl (g)
Outline one difference between the bonding of carbon atoms in C60 and diamond.
State two features showing that propane and butane are members of the same homologous series.
Describe a test and the expected result to indicate the presence of carbon–carbon double bonds.
Draw the full structural formula of but-2-ene.
Write the equation for the reaction between but-2-ene and hydrogen bromide.
State the type of reaction.
Suggest two differences in the 1H NMR of but-2-ene and the organic product from (d)(ii).
Calculate the enthalpy change of the reaction, ΔH, using section 11 of the data booklet.
Draw and label an enthalpy level diagram for this reaction.
This question is about peroxides.
Hydrogen peroxide decomposes to water and oxygen when a catalyst such as potassium iodide, KI, is added.
2H2O2 (aq) O2 (g) + 2H2O (l)
Suggest why many chemicals, including hydrogen peroxide, are kept in brown bottles instead of clear colourless bottles.
In a laboratory experiment solutions of potassium iodide and hydrogen peroxide were mixed and the volume of oxygen generated was recorded. The volume was adjusted to 0 at t = 0.
The data for the first trial is given below.
Plot a graph on the axes below and from it determine the average rate of formation of oxygen gas in cm3 O2 (g) s−1.
Average rate of reaction:
Additional experiments were carried out at an elevated temperature. On the axes below, sketch Maxwell–Boltzmann energy distribution curves at two temperatures T1 and T2, where T2 > T1.
Apart from a greater frequency of collisions, explain, by annotating your graphs in (b)(ii), why an increased temperature causes the rate of reaction to increase.
MnO2 is another possible catalyst for the reaction. State the IUPAC name for MnO2.
Comment on why peracetic acid, CH3COOOH, is always sold in solution with ethanoic acid and hydrogen peroxide.
H2O2 (aq) + CH3COOH (aq) CH3COOOH (aq) + H2O (l)
Sodium percarbonate, 2Na2CO3•3H2O2, is an adduct of sodium carbonate and hydrogen peroxide and is used as a cleaning agent.
Mr (2Na2CO3•3H2O2) = 314.04
Calculate the percentage by mass of hydrogen peroxide in sodium percarbonate, giving your answer to two decimal places.
Xylene is a derivative of benzene. One isomer is 1,4-dimethylbenzene.
Bromine reacts with alkanes.
State the number of 1H NMR signals for this isomer of xylene and the ratio in which they appear.
Number of signals:
Ratio:
Draw the structure of one other isomer of xylene which retains the benzene ring.
Identify the initiation step of the reaction and its conditions.
1,4-dimethylbenzene reacts as a substituted alkane. Draw the structures of the two products of the overall reaction when one molecule of bromine reacts with one molecule of 1,4-dimethylbenzene.
The biochemical oxygen demand of a water sample can be determined by the following series of reactions. The final step is titration of the sample with sodium thiosulfate solution, Na2S2O3 (aq).
2Mn2+ (aq) + O2 (aq) + 4OH− (aq) → 2MnO2 (s) + 2H2O (l)
MnO2 (s) + 2I− (aq) + 4H+ (aq) → Mn2+ (aq) + I2 (aq) + 2H2O (l)
2S2O32− (aq) + I2 (aq) → 2I− (aq) + S4O62− (aq)
A student analysed two 300.0 cm3 samples of water taken from the school pond: one immediately (day 0), and the other after leaving it sealed in a dark cupboard for five days (day 5). The following results were obtained for the titration of the samples with 0.0100 mol dm−3 Na2S2O3 (aq).
Determine the mole ratio of S2O32− to O2, using the balanced equations.
Calculate the number of moles of oxygen in the day 0 sample.
The day 5 sample contained 5.03 × 10−5 moles of oxygen.
Determine the 5-day biochemical oxygen demand of the pond, in mg dm−3 (“parts per million”, ppm).
Calculate the percentage uncertainty of the day 5 titre.
Suggest a modification to the procedure that would make the results more reliable.
The thermal decomposition of dinitrogen monoxide occurs according to the equation:
2N2O (g) → 2N2 (g) + O2 (g)
The reaction can be followed by measuring the change in total pressure, at constant temperature, with time.
The x-axis and y-axis are shown with arbitrary units.
Explain why, as the reaction proceeds, the pressure increases by the amount shown.
Outline, in terms of collision theory, how a decrease in pressure would affect the rate of reaction.
The experiment is repeated using the same amount of dinitrogen monoxide in the same apparatus, but at a lower temperature.
Sketch, on the axes in question 2, the graph that you would expect.
The experiment gave an error in the rate because the pressure gauge was inaccurate. Outline whether repeating the experiment, using the same apparatus, and averaging the results would reduce the error.
The graph below shows the Maxwell–Boltzmann distribution of molecular energies at a particular temperature.
The rate at which dinitrogen monoxide decomposes is significantly increased by a metal oxide catalyst.
Annotate and use the graph to outline why a catalyst has this effect.
Biochemical oxygen demand (BOD) can be determined by the Winkler Method.
A 25.00 cm3 sample of water was treated according to the Winkler Method.
Step I: 2Mn2+ (aq) + O2 (g) + 4OH− (aq) → 2MnO2 (s) + 2H2O (l)
Step II: MnO2 (s) + 2I− (aq) + 4H+ (aq) → Mn2+ (aq) + I2 (aq) + 2H2O (l)
Step III: 2S2O32− (aq) + I2 (aq) → 2I− (aq) + S4O62− (aq)
The iodine produced was titrated with 37.50 cm3 of 5.000 × 10−4 mol dm−3 Na2S2O3.
Outline what is measured by BOD.
A student dissolved 0.1240 ± 0.0001 g of Na2S2O3 to make 1000.0 ± 0.4 cm3 of solution to use in the Winkler Method.
Determine the percentage uncertainty in the molar concentration.
Calculate the amount, in moles of Na2S2O3 used in the titration.
Deduce the mole ratio of O2 consumed in step I to S2O32− used in step III.
Calculate the concentration of dissolved oxygen, in mol dm−3, in the sample.
The three steps of the Winkler Method are redox reactions.
Deduce the reduction half-equation for step II.
When dinitrogen pentoxide, N2O5, is heated the colourless gas undergoes thermal decomposition to produce brown nitrogen dioxide:
N2O5 (g) → 2NO2 (g) + O2 (g)
Data for the decomposition at constant temperature is given.
Suggest how the extent of decomposition could be measured.
Plot the missing point on the graph and draw the best-fit line.
Deduce the relationship between the concentration of N2O5 and the rate of reaction.
Outline why increasing the concentration of N2O5 increases the rate of reaction.
Ethyne, C2H2, reacts with oxygen in welding torches.
Ethyne reacts with steam.
C2H2 (g) + H2O (g) → C2H4O (g)
Two possible products are:
Product B, CH3CHO, can also be synthesized from ethanol.
Write an equation for the complete combustion of ethyne.
Deduce the Lewis (electron dot) structure of ethyne.
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
Identify the type of interaction that must be overcome when liquid ethyne vaporizes.
Product A contains a carbon–carbon double bond. State the type of reactions that compounds containing this bond are likely to undergo.
State the name of product B, applying IUPAC rules.
Determine the enthalpy change for the reaction, in kJ, to produce A using section 11 of the data booklet.
The enthalpy change for the reaction to produce B is −213 kJ. Predict, giving a reason, which product is the most stable.
The IR spectrum and low resolution 1H NMR spectrum of the actual product formed are shown.
Deduce whether the product is A or B, using evidence from these spectra together with sections 26 and 27 of the data booklet.
Identity of product:
One piece of evidence from IR:
One piece of evidence from 1H NMR:
Suggest the reagents and conditions required to ensure a good yield of product B.
Reagents:
Conditions:
Deduce the average oxidation state of carbon in product B.
Explain why product B is water soluble.
Compound A is in equilibrium with compound B.
Predict the electron domain and molecular geometries around the oxygen atom of molecule A using VSEPR.
The IR spectrum of one of the compounds is shown:
COBLENTZ SOCIETY. Collection © 2018 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Deduce, giving a reason, the compound producing this spectrum.
Compound A and B are isomers. Draw two other structural isomers with the formula .
The equilibrium constant, , for the conversion of A to B is in water at .
Deduce, giving a reason, which compound, A or B, is present in greater concentration when equilibrium is reached.
An acidic sample of a waste solution containing Sn2+(aq) reacted completely with K2Cr2O7 solution to form Sn4+(aq).
State the oxidation half-equation.
Deduce the overall redox equation for the reaction between acidic Sn2+(aq) and Cr2O72–(aq), using section 24 of the data booklet.
Calculate the percentage uncertainty for the mass of K2Cr2O7(s) from the given data.
The sample of K2Cr2O7(s) in (i) was dissolved in distilled water to form 0.100 dm3 solution. Calculate its molar concentration.
10.0 cm3 of the waste sample required 13.24 cm3 of the K2Cr2O7 solution. Calculate the molar concentration of Sn2+(aq) in the waste sample.
A 4.406 g sample of a compound containing only C, H and O was burnt in excess oxygen. 8.802 g of CO2 and 3.604 g of H2O were produced.
The following spectrums show the Infrared spectra of propan-1-ol, propanal and propanoic acid.
NIST Mass Spectrometry Data Center Collection © 2021 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved. Available at: https://webbook.nist.gov/cgi/cbook.cgi?ID=C71238&Units=SI&Type=IRSPEC&Index=3#IR-SPEC [Accessed 6 May 2020]. Source adapted.
NIST Mass Spectrometry Data Center Collection © 2021 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. Available at: https://webbook.nist.gov/cgi/cbook.cgi?ID=C79094&Units=SI&Mask=80#IR-Spec [Accessed 6 May 2020]. Source adapted.
NIST Mass Spectrometry Data Center Collection © 2021 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. Available at: https://webbook.nist.gov/cgi/cbook.cgi?Name=propanal&Units=SI&cIR=on&cTZ=on#IRSpec [Accessed 6 May 2020]. Source adapted.
Determine the empirical formula of the compound using section 6 of the data booklet.
Determine the molecular formula of this compound if its molar mass is 88.12 g mol−1. If you did not obtain an answer in (a) use CS, but this is not the correct answer.
Identify each compound from the spectra given, use absorptions from the range of 1700 cm−1 to 3500 cm−1. Explain the reason for your choice, referring to section 26 of the data booklet.